Want to contribute to this article?
ICH E6 R2 is a bible for GCP quality management professionals.
However, it doesn't tell you exactly how to meet any of the requirements. In this article Kaye Eames, Qualsys Training Manager, has shared examples of how EQMS helps you meet the 13 principles for good clinical practice.

Principle 1:
Clinical trials should be conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki that are consistent with GCP and the applicable regulatory requirement(s).
How EQMS helps:
The document control module gives you unshakeable control over your business information and data.
Document ethical principles, policies and commitment statements in a central document control repository. EQMS provides you with a web of information whereby you can link SOPs such as protocols or consent form templates. This provides guidance, but also ensures GCP critical documents are controlled.
By communicating the evaluation process of ethical principles in EQMS, changes, risks or concerns are properly managed through collaborative review, audit trails and a formal approval process.
|
Declaration of Helsinki (basic principles):
|
Principle 2:
Before a trial is initiated, foreseeable risks and inconveniences should be weighed against the anticipated benefit for the individual trial subject and society. A trial should be initiated and continued only if the anticipated benefits justify the risks.
How EQMS helps:
EQMS is a modular quality management software tool used by clinical trial companies across the globe to document and demonstrate GCP
Principle 3:
The rights, safety and well-being of the trial subjects are the most important considerations and should prevail over interest of science and society.
How EQMS helps:
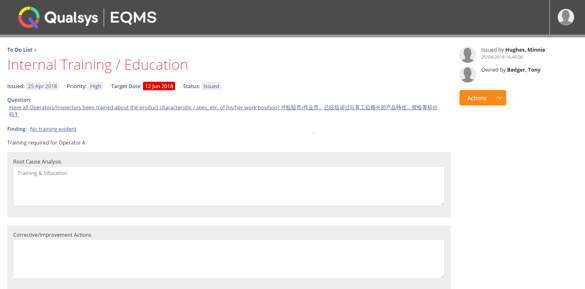
Everything a subject reads or is told needs to be ethically approved. Document Manager ensures that only approved documentation such as SOPs can be viewed. Training Record Manager modules is used to ensure employees are competent and aware of their commitment to trial subject's well-being, safety and rights. Futhermore, Issue Manager is used to capture SOP (standard operating procedure) deviations and adverse events. Advanced permission controls restricts viewing of certain issues and ensures only approved personnel are able to access information of sensitive nature.
New tool: Supplier / customer / subject portals
Controlled EQMS documents can either be accessed securely from EQMS and sent to subjects directly from the system or can be shared through secure subject portals. Within this portal, each trial subject has a unique view of your policies. Subjects have a unique to-do list of documents / training videos / or can raise concerns at any time to ensure proactive care is taken over trial subjects.
Principle 4:
The available non-clinical and clinical information on an investigation product should be adequate to support the proposed clinical trial.
How EQMS helps:
Forms-based modules ensure complete and accurate data is securely captured to ensure the information on an investigation is adequate. Custom metadata enables you to capture any information you need to support the trial.
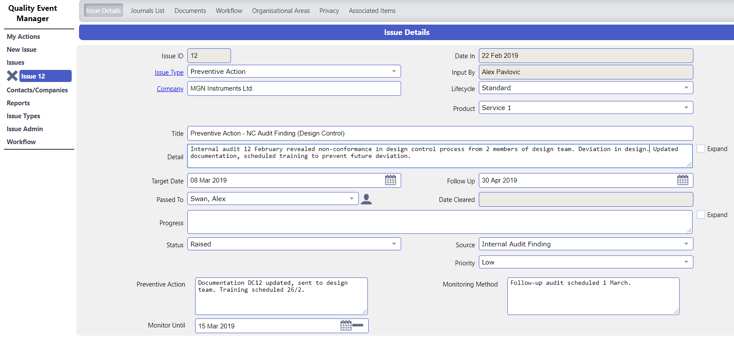
Image: Raising an issue collecting the metadata you need
Principle 5:
Clinical trials should be scientifically sound, and described in clear, detailed protocol.
How EQMS helps:
EQMS provides you with a central repository for storing clear and detailed protocol. All of the protocol templates must be approved through an intelligent workflow tool to ensure the correct approval path has been followed. Furthermore, advanced search algorithms mean you can easily and quickly find the exact information you need.
Principle 6:
A trial should be conducted in compliance with the protocol that has received prior institutional review board (IRB) / independent ethics committee (IEC) approval / favourable opinion.
How EQMS helps:
Audit and Inspection management software enables you to conduct inspections and qualitative audits to ensure protocols are followed.
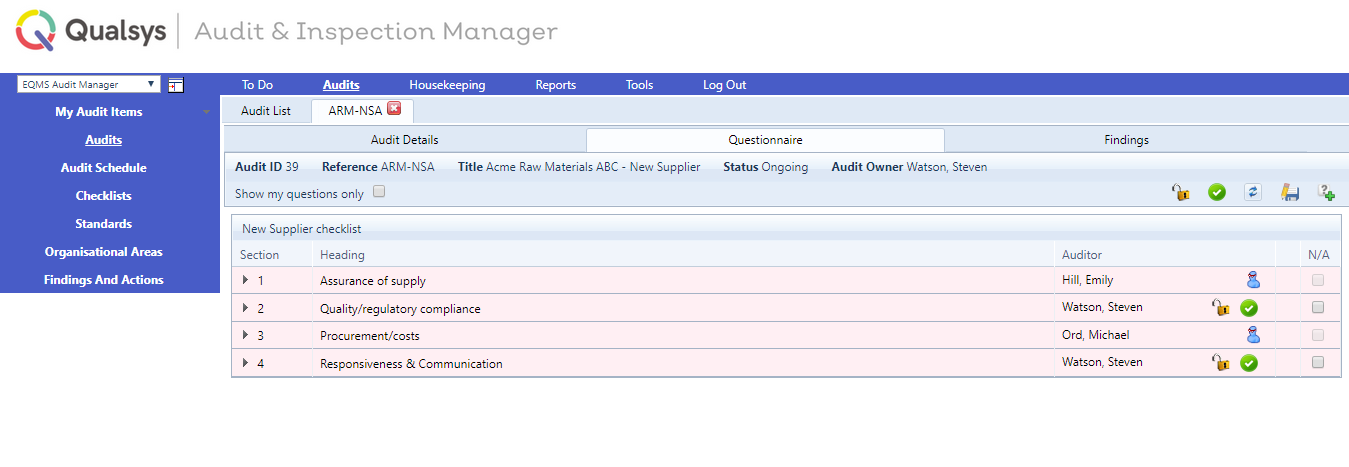
Image: EQMS Audit & Inspection manager
Principle 7:
The medical care given to, and medical decisions made on behalf of subjects should always be the responsibility of a qualified physician or, when appropriate, of a qualified dentist.
How EQMS helps:
Collaborative workflows share the exact information, activities and recommendations from physicians / dentists when they need it. Qualifications associated with the physician or dentist are maintained centrally to demonstrate each person has the appropriate level of training.
Principle 8:
Each individual involved in conducting a trial should be qualified by education, training, and experience to perform his or her respective task(s).
How EQMS helps:
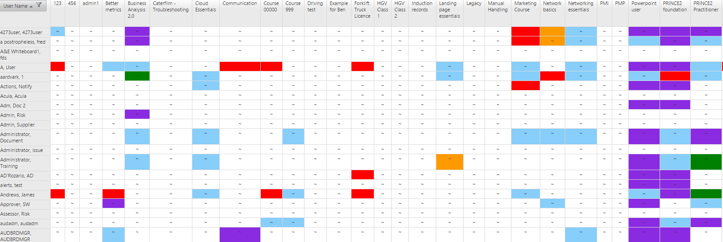
The EQMS Training Record Manager module displays a visual matrix of each employee's qualifications and training record. Authorised personnel can view the status of the training and automatically send training notifications to ensure all employees are competent.
Principle 9:
Freely given informed consent should be obtained from every subject prior to clinical trial participation.
How EQMS helps:
Establish a workflow to ensure consent documents and policies for each clinical trial subject are obtained prior to any test. Then, using Audit Manager, you are able to conduct routine qualitative and quantitative internal audits to ensure consent has been properly obtained and that the processes / SOPs are fit for purpose.
Image: EQMS mobile auditing applications
Principle 10:
All clinical trial information should be recorded, handled, and stored in a way that allows its accurate reporting, interpretation and verification.
How EQMS helps:
EQMS enables you to implement the highest data integrity standards. Data input, processing and reports are protected. There are over 40 live data reports in EQMS - virtually any quality KPI can be recorded and tracked.
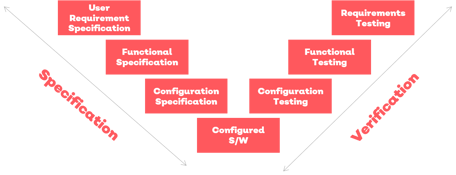
Image: Qualsys validation process aligns with CSV GAMP5 (cat4)
Principle 11:
The confidentiality of records that could identify subjects should be protected, respecting the privacy and confidentiality rules in accordance with the applicable regulatory requirement(s).
How EQMS helps:
EQMS has robust controls for GDPR and ISO 27001 best practices. It is a governance, risk and compliance management system which enables you to protect the confidentiality of subjects through safeguarding data.
Principle 12:
Investigation products should be manufactured, handled and stored in accordance with applicable Good Manufacturing Practice (GMP). They should be used in accordance with the approved protocol.
How EQMS helps:
Supplier Manager module enables you to store all records associated with a supplier. Ensure only approved suppliers and subcontractors are used, maintain their SLAs / policies/ qualifications etc. Supplier Manager also enables you to associate risks, issues, equipment etc. so all information is integrated in a single view.
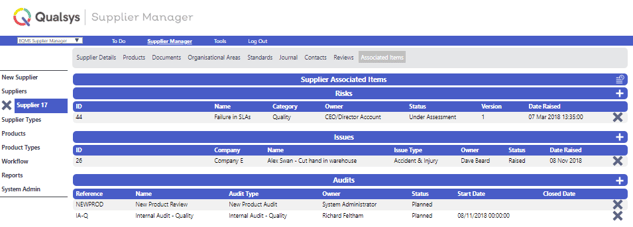
Image: EQMS Supplier Manager
Principle 13:
Systems with procedures that assure the quality of every aspect of the trial should be implemented.
How EQMS helps:
EQMS by Qualsys is a core system for GCP-regulated companies. It enables you to connect and align every procedure across the business. The integrated modules and powerful functionality pull and push data across your business for a true culture of continuous improvement, where critical thinking and a risk-based approach become the norm.
What next?
Join our online webinar "How to align GxP and data integrity for safer products and swifter approvals"








Share your thoughts on this article