Want to contribute to this article?
Most deviations start with data integrity failures. And data integrity failures come at a high cost - from impacting your company’s ability to bring a product to market to causing devastating impacts to end-users.
So, what are some of the top GxP data integrity challenges? What common mistakes should you avoid?
We surveyed 30+ quality practitioners and asked Qualsys Training Manager Kaye Eames to share data integrity compliance challenges, mistakes and tips.
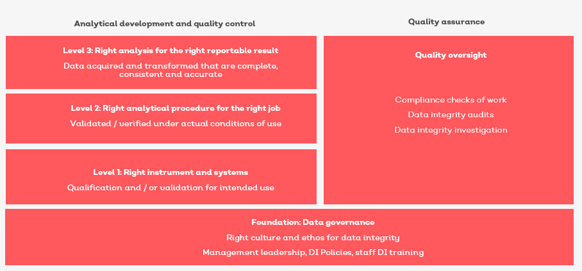 Image: Data integrity framework - requires data governance before you can be confident you have the right analysis
Image: Data integrity framework - requires data governance before you can be confident you have the right analysis
Industry challenges: Soundbites from 30+ quality practitioners
“Changing data integrity culture.”
“Brexit!”
“CSV and data integrity!”
“Manual systems.”
“To avoid making errors and mistakes with data.”
“Regulatory compliance.”
“Quality control”
“Ever changing regulatory environment.”
“Bringing legacy systems up to date and utilising new tools to ensure requirements are met.”
“GxP related processes automation.”
“A paper based manual QMS.”
“Culture in the business.”
"How to get management to take responsibility."
"How to make data integrity and continuous improvement an integrated process."
"Verifying the integrity of the data."
Kaye Eames, Training Manager at Qualsys said:
One of the key challenges when it comes to data integrity is always company culture. If people don't understand why they need to commit to data integrity, they aren't going to do what you tell them to do. We have a whole library full of engagement templates, culture of quality tips, and tools to engage even the most uninterested workforce.
Top seven data integrity mistakes to avoid & how to avoid making them
|
Risk |
Impact |
How EQMS helps |
|
1. Sharing passwords |
Unable to identify who created or changed a records |
Each user has their own password. Electronic signatures comply with FDA and MHRA regulations. |
|
2. User privileges |
System configuration does not segregate user levels |
EQMS promotes horizontal, cross-departmental collaboration. Each person only has access to their own to-do list, notifications and actions. |
|
3. Processing methods |
Integration parameters not controlled |
EQMS enables custom integrations – this means data is migrated how you want instantly between systems. This reduces incorrect manual data entry risk. |
|
4. Audit trails |
Functionality to turn it off – so there is no complete record of the data life cycle |
EQMS audit trails are preserved no matter what. Authorised personnel are able to view a live audit trail pinpointing who, what, when, why, and how a change has taken place. |
|
5. Computer system operational controls |
Unauthorised access to modify or delete files |
EQMS provides unshakeable control over your data, information and reports. Only the latest version of a document may ever be viewed. |
|
6. Failure to review original data |
Data and metadata not reviewed together to ensure context is maintained. |
EQMS brings both your data and metadata into a single source of truth. This ensures errors, anomalies and omissions are detected. |
|
7. Inadequate data retention arrangements |
Failure to avoid inadvertent or deliberate alteration or loss throughout the retention period. |
EQMS enables you to set retention parameters. |
Want to learn how organisations use EQMS to avoid costly data integrity mistakes?
Access our data integrity webinar materials: https://quality.eqms.co.uk/data-integrity-webinar-how-to-align-gxp-and-di









Share your thoughts on this article