Want to contribute to this article?
Medical device manufacturers operate in one of the most heavily regulated business environments on the planet. ISO 13485 and ISO 14791, as well as the requirements of the FDA and MHRA, are just some of the hoops to jump through.
Expanding into international markets only increases the compliance burden.
Fortunately the Medical Device Single Audit Program (MDSAP) offers an accelerated route to market and compliance for medical device companies.

What is the MDSAP?
The MDSAP was formulated in 2012 by the International Medical Device Regulators Forum (IMDRF) as a way of ensuring the appropriate regulatory oversight of the industry in a more efficient and less burdensome manner.
Participating medical device manufacturers can be audited once to demonstrate the necessary standard and regulatory compliance for five international markets.
The participating countries are:
- Australia
- Brazil
- Canada
- Japan
- The United States

The MDSAP opens the door to a combined population of 719.29 million
When did it start?
After a three-year trial period from 1 January 2014 to 31 December 2016, the MDSAP Regulatory Authority Council were sufficiently satisfied to continue the program.
Alongside the five 'equal partners' in the Program, the WHO and EU act as 'Official Observers'. With the successful completion of the trial period a little over a year ago, the possibility for the Program to expand into Europe and beyond shouldn't be ignored.
 When you purchase our software, Qualsys's Service Implementation Team helps you configure our software to meet any regulatory requirements.
When you purchase our software, Qualsys's Service Implementation Team helps you configure our software to meet any regulatory requirements.
What does it do?
The MDSAP's main aims are as follows:
- To minimise regulatory burden by combining five potential audits into one
- To share resources between regulators more efficiently and effectively within a single program
- To promote a greater degree of international standardisation within the medical device industry
Successful completion of the 'single audit' means medical device manufacturers can demonstrate compliance for operation in five territories.
- The Australian Therapeutic Goods Administration will accept MDSAP certificates as evidence of ISO 13485:2003 compliance
- The Brazilian ANVISA will accept MDSAP certification to grant its GMP certificate for manufacturers to put Class III or IV medical devices onto the Brazilian market
- Canada will accept a MDSAP certificate or a Canadian Medical Device Conformity Assessment System (CMDCAS) certificate when granting Class II, III or IV licences.
- Japan's Ministry of Health, Labour & Welfare will use an MDSAP audit report to exempt a manufacturing site from on-site inspection, and may also accept the report in lieu of other documentation required for QMS inspection.
- The American FDA will accept MDSAP audit reports as a substitute for its biennial inspections.

Medical device manufacturers can target five major international markets at once
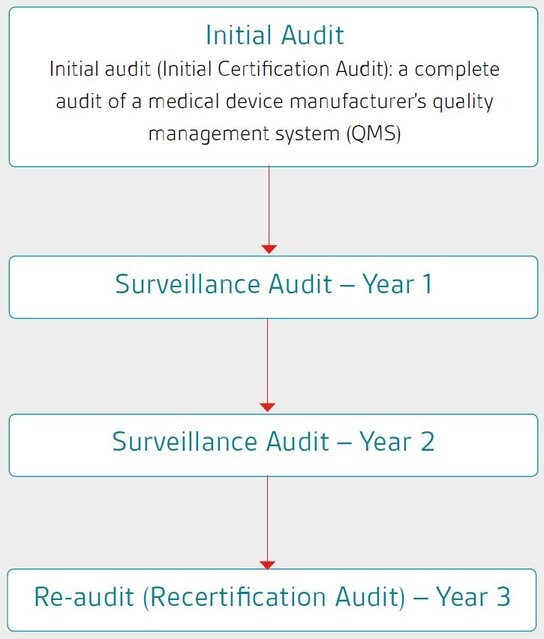
How long does it take?
The Program operates on a three-year audit cycle.
Predictable audit sequences are a further benefit of the Program
How do I take part?
Interested in joining the Program? Find out more at the MDSAP homepage here.
You must contract with an MDSAP-recognised auditing body to arrange your audit. View the complete list of approved organisations here.
Contact one (or more) of the auditing organisations to arrange your audit. MDSAP audits are generally longer than what you might be accustomed to - often between 5 and 9 days.
What should I do next?
If you are planning on entering new markets using MDSAP, our medical device software tool and compliance services helps accelerate the time to market, reduce compliance burden and cost-effectively manage compliance.
Schedule a 15 minute discovery call to learn more.





Share your thoughts on this article